TERAPIA DE REEMPLAZO HORMONAL EN LA MENOPAUSIA Y CANCER
(especial para SIIC © Derechos reservados)La terapia de reemplazo hormonal se asocia con moderada elevación del riesgo de cáncer mamario, pero reduce el de cáncer colorrectal.
Liliane Chatenoud* S Franceschi**
Istituto di Statistica Medica e Biometria, Università degli Studi di Milano, Italia*
**
|
Recepción del artículo 25 de junio, 2003 |
Aprobación 7 de agosto, 2003 |
|
Primera edición 9 de septiembre, 2003 |
Segunda edición, ampliada y corregida 7 de junio, 2021 |
Una de las mayores preocupaciones acerca del uso de la terapia de reemplazo hormonal (TRH) en la menopausia es el efecto de ésta sobre el riesgo de cáncer. La TRH reduce los síntomas climatéricos y ejerce acciones favorables sobre el metabolismo óseo y la osteoporosis. Sin embargo, también ejerce ciertos efectos adversos, entre los que se destacan el efecto promotor sobre el cáncer de endometrio y cierta elevación del riesgo de cáncer de mama y –probablemente- de ovario. Nosotros revisamos las evidencias epidemiológicas en la menopausia acerca de la terapia de reemplazo hormonal y el cáncer, y concluimos que los efectos potencialmente favorables y adversos más marcados de la terapia de reemplazo hormonal (TRH) sobre el riesgo de cáncer se restringen a las usuarias actuales. Los recientes hallazgos de los estudios aleatorizados se encuentran en total acuerdo con los de los estudios de observación (de cohortes y de casos y controles), y proveen evidencias concluyentes de que: (1) la TRH, principalmente la combinación de estrógenos y progestágenos, se asocia con riesgo de cáncer mamario moderadamente elevado, que se hace evidente luego de pocos años de uso, (2) que la TRH combinada no se asocia con elevación de riesgo concreto de cáncer de endometrio y (3) que la TRH ejerce efectos favorables sobre el riesgo de cáncer colorrectal.
Menopausia, hormonas; cáncer de mama; cáncer de endometrio
 Abstract
Abstract
One of the major concerns on hormone replacement therapy (HRT) use in menopause remains the effect on cancer risk. HRT reduces climateric symptoms and has favourable effects on bone metabolism and osteoporosis. HRT, however, also has a number of adverse effects, the main ones being a promotional effect on endometrial cancer, and some elevation in the risk of breast and, possibly, ovarian cancers. We revised the epidemiological evidences on menopause, hormone replacement therapy and cancer, and concluded that most potential favourable and adverse effects on cancer risk of hormone replacement therapy (HRT) are restricted to current users. The recent findings of the randomized trials are in broad agreement with those of observational (cohort and case-control) studies, and provide conclusive evidence that: 1) HRT, mainly combined estrogen/progestagen HRT, is associated with a moderate excess risk of breast cancer, which becomes evident after a few years of use; 2) combined HRT is not associated with a material excess risk of endometrial cancer and 3) HRT has a favourable effect on colorectal cancer risk.
 Key words
Key words
Menopausia, hormonas; cáncer de mama; cáncer de endometrio
(exclusivo a suscriptores)
(english)
Autor
Artículos originales > Expertos del Mundo >
página www.siicsalud.com/des/expertocompleto.php/20046
Especialidades
Principal: Obstetricia y Ginecología
Relacionadas: Farmacología, Obstetricia y Ginecología, Oncología, Salud Pública
Expertos del Mundo
Especialidad principal:
Obstetricia y Ginecología
Relacionadas:
Farmacología
Obstetricia y Ginecología
Oncología
Salud Pública
Hormone Replacement Therapy in Menopause and Cancer
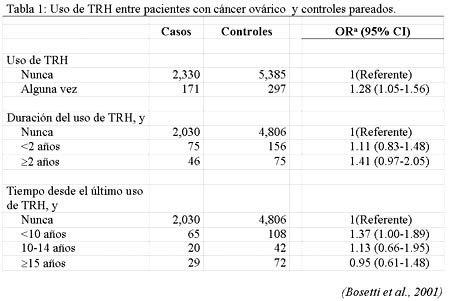
Most potential favourable and adverse effects on cancer risk of hormone replacement therapy (HRT) are restricted to current users. On the basis of observational epidemiological data, the estimation of relative risk (RR) of breast cancer is moderately elevated in current and recent HRT users, and increases by about 2.3% per year with longer duration of use, but the effect drops after cessation and largely, if not totally, disappears after about 5 years. Unopposed estrogen use is strongly related to endometrial cancer risk but cyclic combined oestrogen-progestin treatment appears to largely or totally reduce this side effect, if progestin are used for at least 14 days per cycle. However, combined HRT may be associated with higher risk of breast cancer as compared to unopposed estrogens. HRT has been inversely related to colorectal cancer, although the issue of causal relation remains open to discussion. No consistent association was reported for ovarian, liver, other digestive tract or lung cancer1.In a collaborative analysis of European case-control studies of ovarian cancer2 about 2500 women were included with histologically confirmed epithelial ovarian cancer and 5882 controls enrolled in 5 case-control studies:2 were conducted in Greece, 1 in the United Kingdom, and 1 in Italy between 1979 and 1991, plus another case-control study conducted in 4 Italian locations between 1992 and 1999. In comparison with women who had never used HRT, the odds ratio (OR) for ever users was 1.28 (95% confidence interval (CI), 1.05-1.56). The risk was 1.11 for use less than 2 years and 1.41 for use 2 years or more. With reference to time since last HRT use, the OR was 1.37 for less than 10 years since last use, 1.13 for 10 to 14 years, and 0.95 for 15 or more years since last use (Table 1).  The relation between HRT and gallbladder cancer was analysed in women above age 45 years, using data of a case-control study conducted in Italy between 1985 and 1997, on 31 incident, histologically confirmed cases and 3,702 controls in hospital for acute, non-neoplastic conditions. The multivariate OR was 3.2 (95% CI: 1.1-9.3) for those who had ever used HRT and the OR tended to rise with longer duration3. Between 1979 and 1998, in the Breast Cancer Detection Demonstration Project (BCDDP) cohort study, 329 cases of ovarian cancer were observed4. The relative risk (RR) for estrogen only HRT was 1.6 (95% confidence interval, CI 1.2 to 2.0), forever users, and raised to 1.8 for 10 to 19 years of use, and to 3.2 (95% CI 1.7 to 5.7) for use of =20 years. Data were inadequate to evaluate combined HRT.The major recent information on cancer risk in users of combined (estrogen and progestagen) HRT derives for the Women’s Health Initiative (WHI)5, a randomised controlled primary prevention trial including 8,506 women aged 50 to 70 treated with combined HRT and 8,102 untreated women. The combined treatment was discontinued on 9 July when the US National Institutes of health issued a press release6 stating that the combination hormone medication used in the trial posed more risks than benefits. This was based on increased risks of invasive breast cancer, cardiovascular disease, stroke and blood clots. An additional estrogen compared with placebo only treatment group of the some trial is still ongoing. With reference to cancer risk, data were provided on the incidence of breast, endometrial and colorectal neoplasms. No difference in risk was evident during the first four years after starting treatment, but an excess risk of breast cancer was evident thereafter, as well as a reduced risk of colorectal cancer. Overall, at 7 years follow-up, 166 breast cancer cases were registered |
 Bibliografía del artículo
Bibliografía del artículo- La Vecchia C., Brinton L.A., McTiernan A. Menopause, hormone replacement therapy and cancer. Maturitas 39: 97-115, 2001.
- Bosetti C., Negri E., Franceschi S., Trichopoulos D., Beral V., La Vecchia C. Relationship Between Postmenopausa Hormone Replacement Therapy and Ovarian Cancer. JAMA 285: 3089, 2001.
- Gallus S., Negri E., Chatenoud L., Bosetti C., Franceschi S., La Vecchia C. Post-menopausal hormonal therapy and gallbladder cancer risk. Int. J. Cancer 99: 762-763, 2002.
- Lacey JV Jr, Mink PJ, Lubin JH, Sherman ME, Troisi R, Hartge P, Schatzkin A, Schairer C. Menopausal hormone replacement therapy and risk of ovarian cancer. JAMA 288: 334-341, 2002.
- Writing Group for the Women’s Health Initiative Investigators. Risks and benefits of estrogen plus progestin in healthy postmenopausal women: principal results from the Women’s Health Initiative randomized controlled trial. JAMA 288: 321-333, 2002.
- Digest Page: Postmenopausal Hormone Use.http://www.nci.nih.gov/clinical_trials
- Hulley S, Furberg C, Barrett-Connor E, Cauley J, Grady D, Haskell W, Knopp R, Lowery M, Satterfield S, Schrott H, Vittinghoff E, Hunninghake D, HERS Research Group. Noncardiovascular disease outcomes during 6.8 years of hormone therapy: Heart and Estrogen/progestin Replacement Study follow-up (HERS II). JAMA 288: 58-66, 2002.
- Viscoli CM, Brass LM, Kernan WN, Sarrel PM, Suissa S, Horwitz RI. A clinical trial of estrogen-replacement therapy after ischemic stroke. N Engl J Med 345: 1243-1249, 2001.
- Beral V, Banks E, Reeves G. Evidence from randomised trials on the long-term effects of hormone replacement therapy. Lancet 360: 942-44, 2002.
- La Vecchia C, Franceschi S. Hormone replacement therapy and cancer: an update. Eur J Cancer Prev 12:3-4, 2003.
Extensión: ± 2.43 páginas impresas en papel A4
Está expresamente prohibida la redistribución y la redifusión de todo o parte de los contenidos de la Sociedad Iberoamericana de Información Científica (SIIC) S.A. sin previo y expreso consentimiento de SIIC.


































